- Topic1/3
51k Popularity
8k Popularity
7k Popularity
2k Popularity
658 Popularity
- Pin
- #Gate 2025 Semi-Year Community Gala# voting is in progress! 🔥
Gate Square TOP 40 Creator Leaderboard is out
🙌 Vote to support your favorite creators: www.gate.com/activities/community-vote
Earn Votes by completing daily [Square] tasks. 30 delivered Votes = 1 lucky draw chance!
🎁 Win prizes like iPhone 16 Pro Max, Golden Bull Sculpture, Futures Voucher, and hot tokens.
The more you support, the higher your chances!
Vote to support creators now and win big!
https://www.gate.com/announcements/article/45974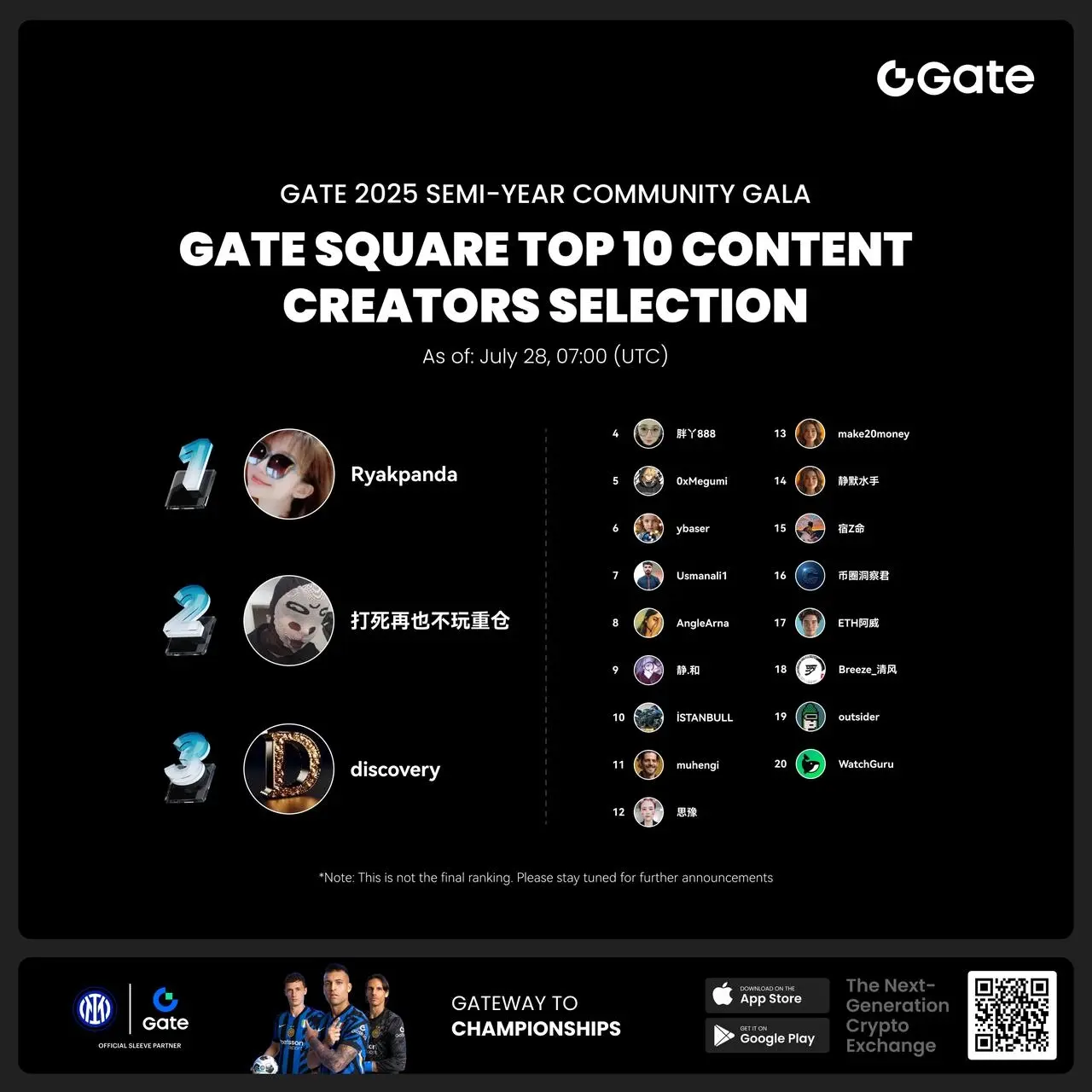
- 🎉 Hey Gate Square friends! Non-stop perks and endless excitement—our hottest posting reward events are ongoing now! The more you post, the more you win. Don’t miss your exclusive goodies! 🚀
1️⃣ #ETH Hits 4800# | Market Analysis & Prediction: Boldly share your ETH predictions to showcase your insights! 10 lucky users will split a 0.1 ETH prize!
Details 👉 https://www.gate.com/post/status/12322612
2️⃣ #Creator Campaign Phase 2# |ZKWASM Topic: Share original content about ZKWASM or its trading activity on X or Gate Square to win a share of 4,000 ZKWASM!
Details 👉 https://www.gate.com/post/st - 📢 Gate Square #Creator Campaign Phase 2# is officially live!
Join the ZKWASM event series, share your insights, and win a share of 4,000 $ZKWASM!
As a pioneer in zk-based public chains, ZKWASM is now being prominently promoted on the Gate platform!
Three major campaigns are launching simultaneously: Launchpool subscription, CandyDrop airdrop, and Alpha exclusive trading — don’t miss out!
🎨 Campaign 1: Post on Gate Square and win content rewards
📅 Time: July 25, 22:00 – July 29, 22:00 (UTC+8)
📌 How to participate:
Post original content (at least 100 words) on Gate Square related to
The first three common De-Da-Botu monoclonal antibody has been approved in Japan for the treatment of breast cancer.
On December 28, Jinshi Data reported that on December 27, local time, The First Three Company announced that the targeted TROP2 antibody-drug conjugate (ADC) DATROWAY® (trastuzumab deruxtecan) developed by the company in collaboration with AstraZeneca has been approved in Japan for the treatment of adult patients with unresectable or recurrent breast cancer who are hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-), and have an immunohistochemistry (IHC) score of 0, 1+, or 2+ with in situ hybridization (ISH-) after receiving chemotherapy.